How to Draw Hydroxide Lewis Structure: Easy Guide
<!DOCTYPE html>
Drawing the Lewis structure of hydroxide (OH-) is a fundamental skill in chemistry, especially for understanding its role in various chemical reactions. Whether you’re a student or a chemistry enthusiast, this guide will walk you through the process step-by-step. By the end, you’ll be able to confidently draw the hydroxide Lewis structure and understand its significance. (Lewis structure, hydroxide ion, chemical bonding)
What is the Hydroxide Ion (OH-)?

The hydroxide ion (OH-) is a negatively charged ion consisting of one oxygen atom and one hydrogen atom. It plays a crucial role in acid-base chemistry, often acting as a base in aqueous solutions. Understanding its Lewis structure helps in predicting its reactivity and behavior in chemical reactions. (hydroxide ion, acid-base chemistry, chemical reactions)
Step-by-Step Guide to Drawing the Hydroxide Lewis Structure

Step 1: Determine the Total Number of Valence Electrons
Oxygen has 6 valence electrons, hydrogen has 1, and the negative charge adds 1 more electron. So, the total number of valence electrons is 8. (valence electrons, electron configuration)
Step 2: Choose the Central Atom
In the hydroxide ion, oxygen is the central atom because it is more electronegative than hydrogen. (central atom, electronegativity)
Step 3: Connect the Atoms with Single Bonds
Draw a single bond between the oxygen and hydrogen atoms. This uses up 2 electrons, leaving 6 electrons to be distributed. (single bond, electron distribution)
Step 4: Complete the Octet on the Central Atom
Place the remaining 6 electrons around the oxygen atom to complete its octet. These electrons are represented as lone pairs. (octet rule, lone pairs)
📌 Note: Hydrogen can only have 2 electrons in its outermost shell, which is already satisfied by the single bond with oxygen.
Step 5: Verify the Structure
Ensure that all atoms have the correct number of electrons and that the total number of valence electrons is accounted for. The hydroxide ion should have a complete octet for oxygen and a duet for hydrogen. (Lewis structure verification, octet rule)
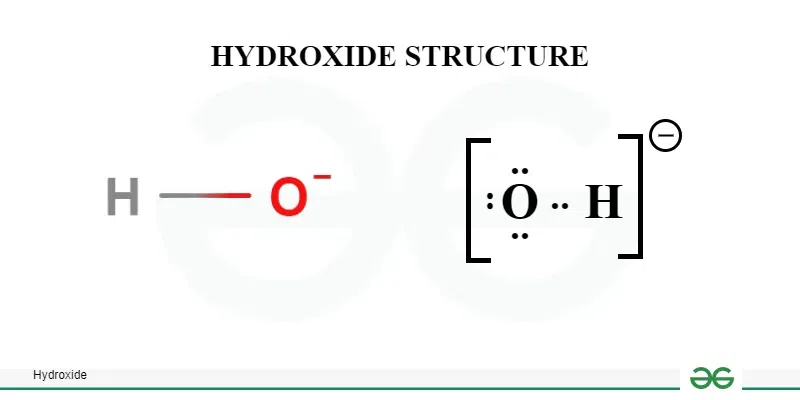
Hydroxide Lewis Structure: Visual Representation

The final Lewis structure of the hydroxide ion (OH-) is:
O- - H (with three lone pairs on the oxygen atom). (Lewis structure diagram, chemical bonding)
Checklist for Drawing the Hydroxide Lewis Structure

- Calculate the total number of valence electrons.
- Identify the central atom (oxygen in this case).
- Draw a single bond between oxygen and hydrogen.
- Place lone pairs on the oxygen atom to complete its octet.
- Verify the structure to ensure all electrons are accounted for.
Mastering the hydroxide Lewis structure is essential for understanding its role in chemical reactions and its significance in acid-base chemistry. With this easy guide, you’re now equipped to draw it accurately. (acid-base chemistry, chemical reactions, Lewis structure)
What is the hydroxide ion used for?
+The hydroxide ion (OH-) is crucial in acid-base reactions, often acting as a base in aqueous solutions. It also plays a role in neutralization reactions and maintaining pH balance. (hydroxide ion, acid-base reactions, pH balance)
Why does oxygen act as the central atom in OH-?
+Oxygen acts as the central atom because it is more electronegative than hydrogen and can form more bonds and hold more electrons. (central atom, electronegativity, chemical bonding)
How many lone pairs are on the oxygen atom in OH-?
+There are three lone pairs on the oxygen atom in the hydroxide ion (OH-). (lone pairs, oxygen atom, Lewis structure)



